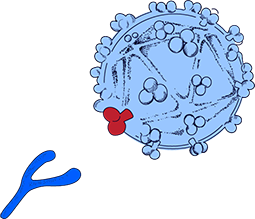
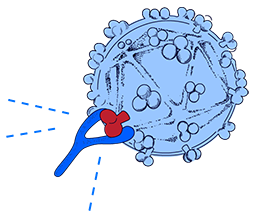
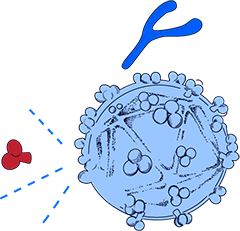
How our HIV Vaccine Works
HIV is difficult to treat and vaccinate against because it is constantly mutating, changing faster than the antibodies our body produces to fight it.
Dr. Sudhir Paul has invented an E-Vaccine that allows the immune system to attack a mostly unchangeable and thus vulnerable part of HIV.